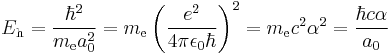Hartree
The hartree (symbol: Eh or Ha), also known as the Hartree energy, is the atomic unit of energy, named after the British physicist Douglas Hartree. It is defined as 2R∞hc, where R∞ is the Rydberg constant, h is the Planck constant and c is the speed of light. The 2006 CODATA recommended value is Eh = 4.359 743 94(22)×10−18 J = 27.211 383 86(68) eV.[1]
The hartree energy is approximately the electric potential energy of the hydrogen atom in its ground state and, by the virial theorem, approximately twice its ionization energy; the relationships are not exact because of the finite mass of the nucleus of the hydrogen atom and relativistic corrections.
The hartree is usually used as a unit of energy in atomic physics and computational chemistry: for experimental measurements at the atomic scale, the electronvolt (eV) or the reciprocal centimetre (cm−1) are much more widely used.
Other relationships
where:
- ħ is the reduced Planck constant,
- me is the electron rest mass,
- e is the elementary charge,
- a0 is the Bohr radius,
- ε0 is the electric constant,
- c is the speed of light in vacuum, and
- α is the fine structure constant.
References
- ^ Mohr, Peter J.; Taylor, Barry N.; Newell, David B. (2008). "CODATA Recommended Values of the Fundamental Physical Constants: 2006". Rev. Mod. Phys. 80: 633–730. Bibcode 2008RvMP...80..633M. doi:10.1103/RevModPhys.80.633. http://physics.nist.gov/cuu/Constants/codata.pdf. Direct link to value..